Table Of Content
This ensures a smooth transition for experienced colorists while offering a user-friendly introduction for beginners. Case Design Corporation (CDC) is a privately owned and operated business serving the industry for over 90 years. The solution provides the ability to collect evidence once and map it across multiple regulations – simplifying compliance to multiple regulations.
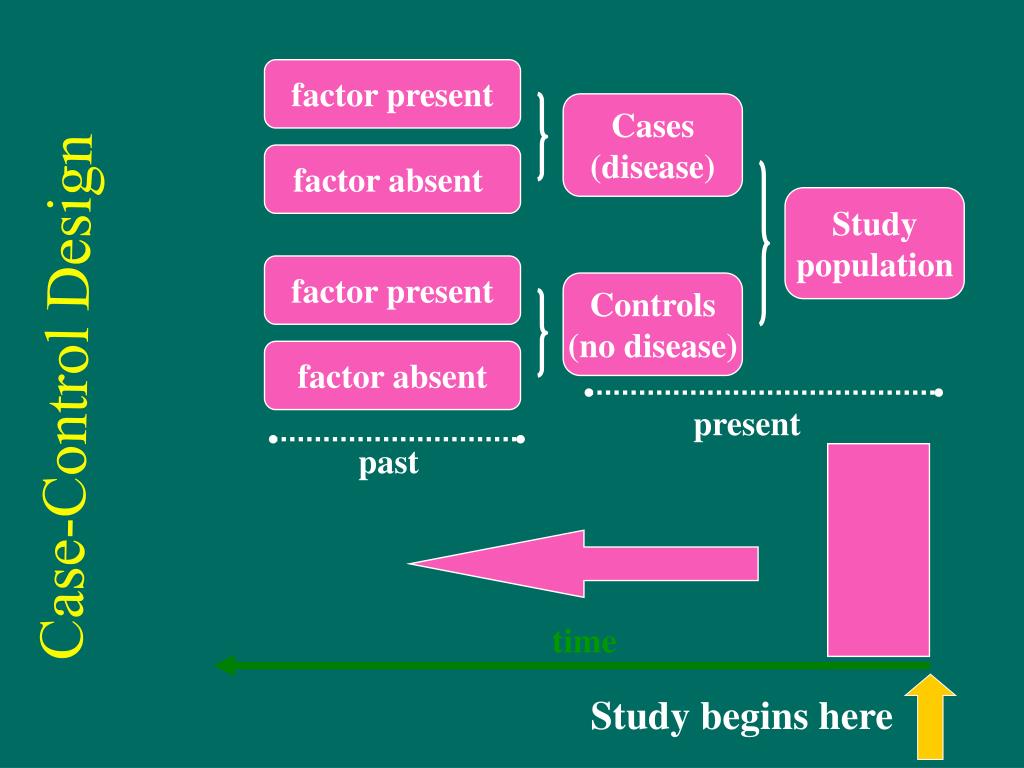
Case Control Studies
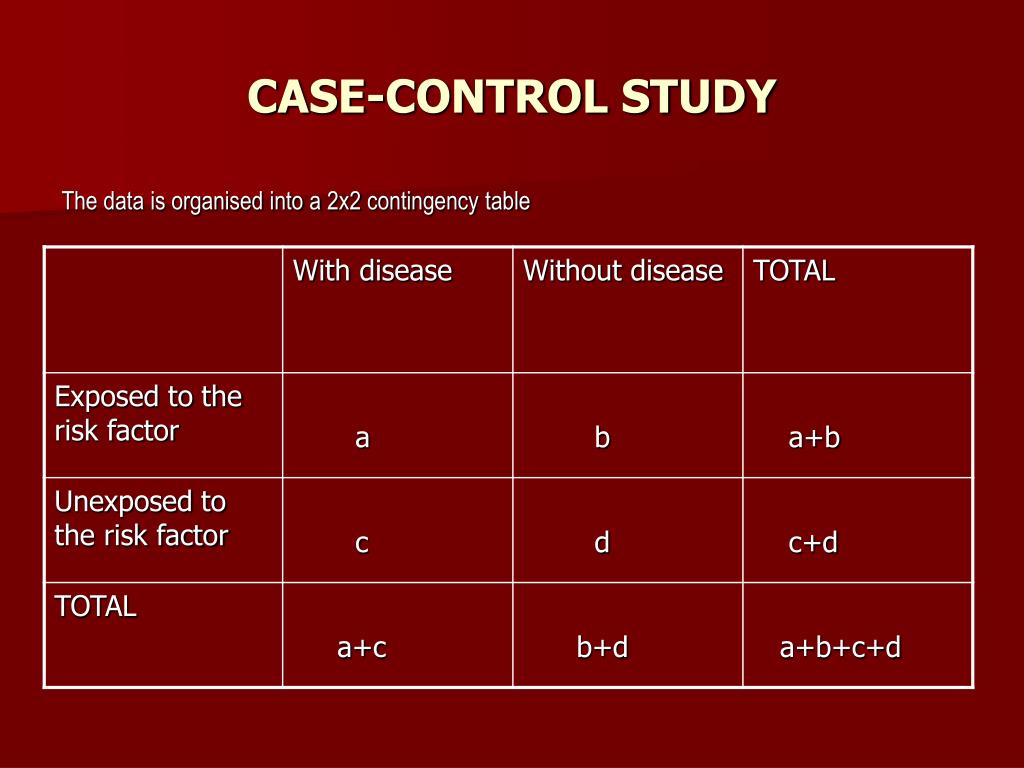
There are of course many more causal contrasts, treatment regimes and estimands conceivable that could be of interest. We argue that also for these estimands, researchers should seek to establish identifiability before they select an estimator. Table 2 gives an overview of identification results for case-control studies with exact pair matching.
Case-control studies with matching
Case-control studies are different from cross-sectional studies in that case-control studies compare groups retrospectively while cross-sectional studies analyze information about a population at a specific point in time. Forming an accurate control group can be challenging, so sometimes researchers enroll multiple control groups to bolster the strength of the case-control study. If the exposure is found more commonly in the cases than the controls, the researcher can hypothesize that the exposure may be linked to the outcome of interest. Case Control Studies are prospective in that they follow the cases and controls over time and observe what occurs.
An Introduction to the Fundamentals of Cohort and Case–Control Studies
During the analysis of study data, multivariate analysis (usually logistic regression) can be used to adjust for the effect of measured confounders. A cohort study compares the experience of 2 or more groups of patients who are followed concurrently forward in time (Figure 1). While one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. In particular, retrospective cohort studies are designed to follow a group of people with a common exposure or risk factor over time and observe their outcomes. Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology.
Study Designs in the Health Sciences
Wacholder and colleagues (1992 a, b, and c) have published wonderful manuscripts on design and conduct of case-control of studies in the American Journal of Epidemiology. The data were collected by self administered questionnaires and telephone interviews. The investigators assessed the use of tanning devices (using photographs), number of years, and frequency of use of these devices. They also collected information on other variables (such as sun exposure; presence of freckles and moles; and colour of skin, hair, among other exposures. Supplementary material to ‘Identification of causal effects in case-control studies’.
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. A case-control study of readmission to the intensive care unit after cardiac surgery. In addition, case-control studies look at a single subject or a single case, whereas longitudinal studies can be conducted on a large group of subjects.
Clinical expertise is essential for developing exposure and outcome definitions, as well as for understanding the overall clinical context of how the research question fits into the current body of knowledge. Methodologic expertise is critical for ensuring that robust methods are used, to minimize bias and confounding. Similar to the situation for a cohort study, the drug exposures of interest and their definitions should be clearly specified in the methods. Because exposure in a case–control study is determined after the cases have been identified, a period before occurrence of the case, called the “look-back period” or “look-back window”, must be defined. Look-back periods should consider the study hypothesis and thus may vary considerably from one study to another.
What is a case-control study?
The case group would consist of all those patients at the hospital who developed post-operative endophthalmitis during a pre-defined period. You can cite our article (APA Style) or take a deep dive into the articles below. This would allow you to observe whether the people exposed to the chemical had more instances of mesothelioma than those who weren’t exposed. Compare your paper to billions of pages and articles with Scribbr’s Turnitin-powered plagiarism checker.
Explaining Recruitment to Extremism: A Bayesian Hierarchical Case-Control Approach - The Washington Institute
Explaining Recruitment to Extremism: A Bayesian Hierarchical Case-Control Approach.
Posted: Thu, 16 Nov 2023 08:00:00 GMT [source]
If care is taken with definitions, selection of controls, and reducing the potential for bias, case-control studies can generate valuable information. One should not use the word case-control study for a randomised controlled trial (even though you have a control group in the study). For a study to be classified as a case-control study, the study should be an observational study and the participants should be recruited based on their outcome status (some have the disease and some do not).
The counterfactual framework and emulation approach have become increasingly popular in observational cohort studies. A notable exception is given by Dickerman et al. [3], who recently outlined an application of trial emulation with case-control designs to statin use and colorectal cancer. It is our hope that a better understanding of the case-control study design and how it differs from other observational studies will promote improved quality in future reporting within the neurosurgical community. Additionally, a better understanding of the case-control study design will enable the clinician to be more critical when interpreting the significance of reported results. Researchers in case-control studies start with a population of people known to have the target disease instead of following a population and waiting to see who develops it. This enables researchers to identify current cases and enroll a sufficient number of patients with a particular rare disease.
The controls should have similar characteristics (i.e., age, sex, demographic, health status) to the cases to mitigate the effects of confounding variables. Finally, case-control studies, like cohort studies, are observational in nature, andauthors who conduct and report such studies should follow the Strengthening the Reporting ofObservational Studies in Epidemiology (STROBE) guidelines. Because of these advantages, case-control studies are commonly used as one of the first studies to build evidence of an association between exposure and an event or disease. The authors conducted a case-control study to study the association between melanoma and tanning.
Case-control studies are advantageous because they require smaller sample sizes and thus fewer resources and less time than other observational studies. The case-control design also is the most practical option for studying exposure related to rare diseases. Case-control studies also are used for diseases that have long latent periods (long durations between exposure and disease manifestation) and are ideal when multiple potential risk factors are at play. Pharmacists use knowledge from cohort and case–control studies to inform patients, clinicians, and the general public about drug effects. At a basic level, cohort and case–control studies quantitatively estimate the relation between exposures and outcomes. They represent rigorous study designs for answering drug safety and effectiveness questions, with case–control studies being more prone to bias.
No comments:
Post a Comment